Structure – Early work
Alginic acid was discovered, first extracted and patented by Stanford in the 1880’s (Stanford 1881 and 1885). The patent explains how alginate can be extracted by soaking seaweed in water or dilute acid, extracting with sodium carbonate and then precipitating the alginate out of solution with acid. Stanford described alginate as containing nitrogen, but this can be attributed to protein impurities and is not due to the alginate itself (Krefting, 1896 and 1898; Hoagland and Lieb, 1915).
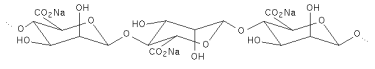
Figure: Sodium polymannuronic acid
Krefting (Krefting, 1896 and 1898) was the first to prepare a purified form of alginic acid but it was not until 1930 that the first structure of alginic acid was proposed (Nelson and Cretcher 1929 and 1930). Alginate was heated in acid and the pentoses formed by the loss of carbon dioxide were isolated and identified by osazone formation. The osazone formed could only have come from D-xylose or D-lyxose and consequently the uronic acids possible could only have been D-glucuronic acid, D-iduronic acid, D-Mannuronic acid and L-guluronic acid. Ironically the presence of guluronic or iduronic acid was excluded on the basis that they had never been found in nature before. Glucuronic acid was ruled out experimentally. The structure proposed was that alginate consisted of a polymeric uronic acid consisting largely of D-mannuronic acid residues. This was established by comparing the diamide and diphenyl hydrazide derivatives of the uronic acid isolated from the polymer with analogous derivatives prepared from D-mannurono-6,3-lactone. They also showed that, on heating in acid, carbon dioxide was released from the polysaccharide and that the weight loss was consistent with alginate being a polyuronate structure.
The methylation work of Hirst and his co-workers showed the polymer to be essentially made up of 1:4 linked β-D-mannuronic acid residues (Hirst 1939). It wasn’t until 1955 that, by paper chromatography, L-guluronic acid was also detected as a major component of alginate (Fischer and Darfel, 1955). They also demonstrated that the ratio of of L-guluronic acid to D-mannuronic acid was varied depending on the species of seaweed used. Fractional precipitation with potassium chloride (Haug, 1959), manganese salts (McDowell R H, 1958) or a combination of magnesium and calcium salts (Haug and Smidsrod, 1965) leads to a concentration of the two uronides into separate fractions. By partial acid hydrolysis and isolation of the oligomers it was shown that mannosyl gulose was present in the hydrolysis products of reduced alginic acid and hence at least some molecules of alginate contained both uronic acids (Vincent 1960; Hirst, Percival and Wold 1964).
Additional information on alginate structure, production and bacterial alginate can be accessed using the arrows in the Further Reading box below.
Further Reading
Read more on Alginate
