Structure – M/G ratios
The usual method for the determination of the ratio of the two uronic acid types is by hydrolysis of the chain followed by analysis of the sugar components. This has inherent problems in that under acid hydrolysis conditions the monomers are subject to degradation via decarboxylation and the rates of degradation for the two uronic acids are not equal; guluronic acid degrading faster than mannuronic acid (Knutson and Jeanes, 1968). Also the rates of hydrolysis depends on the actual distribution of the residues along the chain (Grasdalen, 1979). Once hydrolysed the monomers were separated on an ion exchange column with gradient elution of increasing acetic acid concentration and the residues were subsequently detected using a standard assay such as the carbazole assay (Haug and Larsen, 1962). There have been several modifications to this procedure; the column eluent has been changed to tris-acetate buffer (Spiro, 1977), sodium acetate (Mopper, 1978) and a mixed borate / phosphate buffer (Simatupang, 1979). Alternatives to the assay procedure have also been investigated (Blumenkrantz and Asboe-Hansen, 1973; Wardi, 1974 and Mopper 1978).
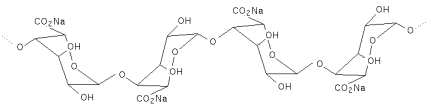
A semi quantitative determination of the ratios of the two residues is possible by comparing the peak areas in the infra red spectrum at 808cm-1 for β-D-mannuronic acid and 887cm-1 for α-L-guluronic acid (Mackie, 1971).
A polarimetric method involves the hydrolysis of alginate followed by measuring the optical rotation of the brucine (10,11-dimethoxystrychnine) salt of the free acids, once again this method suffers from the degradation of the monomers during hydrolysis (Siddiqui, 1978).
Electrophoresis has been used for the separation of hydrolysed alginates using free boundary methods (Haug, 1967), polyacrylamide gels (Bucke, 1974) and agarose gels (Vreeland, 1978).
Another method uses partial hydrolysis, esterification with EDC followed by sodium borohydride reduction and further acid hydrolysis to give D-mannose and L-gulose. Further reduction gives D-mannitol and D-glucitol which are then assayed as their n-butyl boronic esters (Vadas, 1981).
HPLC has proved very popular in the analysis of uronic acid ratios in alginate largely due to its convenience and rapidity of use (Cheetham and Sirimanne, 1983; Honda, 1983). One method involved the methanolysis of alginic acid followed by analysis on an HPLC column with methanolysed D-mannurono-6,3-lactone as a standard. The samples were analysed by NMR (Grasdalen, 1979) but the method appears to over estimate the mannuronic acid content, probably due to incomplete methanolysis, the mannuronic acid residues reacting preferentially to the guluronic acid residues (Annison, 1983). Underivatised alginate hydrolysate has been analysed on an ion exchange column with UV detection (Gaseca, 1983).
Additional information on alginate structure, production and bacterial alginate can be accessed using the arrows in the Further Reading box below.
Further Reading
Read more on Alginate
