Structure – Poly G
The polyguluronic acid segments are very different to the polymannuronic acid segments. The guluronic acid residues are in the 1C4 conformation and are therefore diaxially linked along the polymer chain. This gives the ribbon structure of the polymer a buckled, as opposed to flat, conformation. This structure is stabilised by a different set of hydrogen bonds; the hydroxyl group on C2 of one residue hydrogenbonds with the carboxyl residue in an adjacent residue. The interchain bonding involves water molecules and is considerably more complex than that seen for the poly mannuronic acid residues. This study also gave the first evidence for the L-configuration of the guluronic acid residues. (Atkins et al, 1970; 1971, 1973a, 1973b).
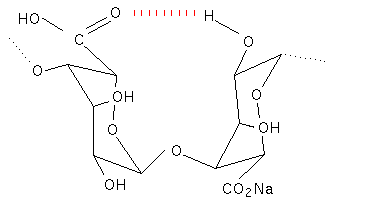
Proton NMR has helped to substantiate the X-ray work and has also been used to determine the ratio’s of the three different types of polymer segment in commercial samples of alginate (Penman & Sanderson, 1972). The two different conformations found in polyguluronic acid and polymannuronic acid oligomers could also explain the differences in their ultra violet dichroism traces (Morris, 1973).
The difference in the block copolymer structure of various types of seaweeds explains why alginate with differing properties is extracted from different seaweeds (Haug, 1967; Fisher and Darfel, 1955). It has ben shown that the conditions of growth of the weed affects the block composition as does the part of the weed from which the alginate is extracted from (Haug et al, 1968).
Additional information on alginate structure, production and bacterial alginate can be accessed using the arrows in the Further Reading box below.
Further Reading
Read more on Alginate
