Xanthan has a particularly complicated molecular structure. The backbone is a β-(1-4)-D-glucose which is the same as cellulose, but every alternate glucose reside has a three sugar side chain consisting of two mannose residues with a glucuronic acid residue between them. The mannose reside nearest the main chain can carry a C6 acetyl group and the terminal mannose can carry a pyruvate group between C4 and C6. The level of acetylation and/or pyruvylation varies depending on fermentation conditions but typically, pyruvate residues are found on 30-40% of the terminal mannose residues and 60-70% of the internal mannose residues contain acetate groups.
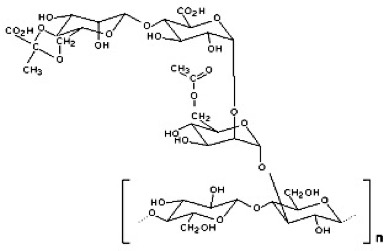
Xanthan is produced in its native state as a twin stranded, right handed fivefold helix. The complexity of the side chains give protection and makes Xanthan very stable under wide range of conditions including acidic, alkaline, high salt concentrations and also to shear and enzymatic hydrolysis. Xanthan is also stable to a range of temperatures but it’s properties change. On heating the xanthan helix goes through a transition to a disordered state and upon cooling it reverts to a helical structure. However, it is believed that native xanthan exists in a form where chains are paired and once that has been lost and the xanthan molecules allowed to reorder the exact pairing cannot be retained and a partially crosslinked structure is formed as helices twist around various neighbours.
Xanthan gum does not gel on its own but shows some gelling synergy with other galacto- and glucomannan gums. These gums also give a synergistic increase in viscosity.
Additional information on xanthan which can be accessed using the arrows in the Further Reading box below.
