Prepared at the 49th JECFA (1997), published in FNP 52 Add 5 (1997) superseding specifications prepared at the 44th JECFA (1995), published in FNP 52 Add 3 (1995). An ADI ‘not specified’ was established at the 39th JECFA (1992)
SYNONYMS
INS No. 404
DEFINITION
Calcium salt of alginic acid.
C.A.S. number
9005-35-0
Chemical formula
(C6 H7 Ca1/2 O6)n
Structural formula
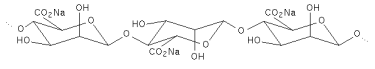

The number and sequence of the Mannuronate and Glucuronate residues shown above vary in the naturally occurring alginate. The associated water molecules are not hown.
Formula weight
Structural unit : 195.16 (theoretical), 219 (actual average)
Macromolecule: 10,000 – 600,000 (typical average)
Assay
Not less than 18.0% and not more than 21.0% of carbon dioxide (CO2), equivalent to not less than 89.6% and not more than 104.5% of calcium alginate (C6H7Ca1/2 O6)n on the anhydrous basis.
DESCRIPTION
White to yellowish brown filamentous, grainy, granular and powdered forms
FUNCTIONAL USES
Stabilizer, thickener, gelling agent, emulsifier
CHARACTERISTICS
IDENTIFICATION
Solubility
Insoluble in water and ether; slightly soluble in ethanol; slowly soluble in solutions of sodium polyphosphate, sodium carbonate, and substances that combine with calcium ions.
Precipitate formation with calcium chloride
To a 0.5% solution of the sample in sodium hydroxide TS add one-fifth of its volume of a 2.5% solution of calcium chloride. A voluminous, gelatinous precipitate is formed. This test distinguishes calcium alginate from gum arabic, sodium carboxymethyl cellulose, carrageenan, gelatin, gum ghatti, karaya gum, carob bean gum, methyl cellulose and tragacanth gum.
Precipitate formation with with ammonium sulfate
To a 0.5% solution of the sample in sodium hydroxide TS add one-half of its volume of a saturated solution of ammonium sulfate. No precipitate is formed. This test distinguishes calcium alginate from agar, sodium carboxymethyl cellulose, carrageenan, de-esterified pectin, gelatin, carob bean gum, methyl cellulose and starch.
Test for alginate
Passes test.
Dissolve as completely as possible 0.1 g of sample by shaking with 0.15 ml of 0.1 N sodium hydroxide and add 1 ml of acid ferric sulfate TS. Within 5 min, a cherry-red colour develops that finally becomes deep purple.
Calcium
Passes test
PURITY
Loss on drying
Not more than 15% (105o, 4 h)
Arsenic
Not more than 3 mg/kg (Method II)
Lead
Not more than 5 mg/kg
Determine using an atomic absorption technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on the principles of the method described in Volume 4, “Instrumental Methods.”
Microbiological criteria
Total plate count: Not more than 5,000 colonies per gram.
Initially prepare a 10-1 dilution by adding a 50 g sample to 450 ml of Butterfield’s phosphate buffered dilution water and homogenizing in a high speed blender.
Yeasts and moulds: Not more than 500 colonies per gram
Coliforms: Negative by test
Salmonella: Negative by test
METHOD OF ASSAY
Proceed as directed under Carbon Dioxide Determination by Decarboxylation in the General Methods. Each ml of 0.25 N sodium hydroxide consumed is equivalent to 5.5 mg of carbon dioxide (CO2) or 27.38 mg of calcium alginate (equivalent weight 219).
