| Prepared at the 49th JECFA (1997), published in FNP 52 Add 5 superseding specifications prepared at the 44th JECFA (1995), published in FNP52 Add 3 (1995). Metals and arsenic specifications revised at the 57th JECFA (2001). An ADI ‘not specified’ was established at the 39th JECFA (1992) | |
| SYNONYMS | INS No. 403 |
| DEFINITION | Ammonium salt of alginic acid. |
| C.A.S. number | 9005-34-9 |
| Chemical formula | (C6 H11 NO6)n |
| Structural formula | 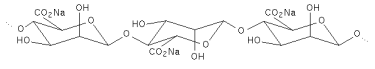  The number and sequence of the Mannuronate and Glucuronate residues shown above vary in the naturally occuring alginate. The associated water molecules are not shown. The number and sequence of the Mannuronate and Glucuronate residues shown above vary in the naturally occuring alginate. The associated water molecules are not shown. |
| Formula weight | Structural unit : 193.16 (theoretical), 217 (actual average)Macromolecule : 10,000 – 600,000 (typical average) |
| Assay | Yields, on the dried basis, not less than 18.0% and not more than 21.0% of carbon dioxide (CO2), equivalent to not less than 88.7% and not more than 103.6% of ammonium alginate C6H11 NO6)n. |
| DESCRIPTION | White to yellowish brown filamentous, grainy, granular or powdered forms |
| FUNCTIONAL USES | Stabilizer, thickener, gelling agent, emulsifier |
| CHARACTERISTICS | |
| IDENTIFICATION | |
| Solubility | Dissolves slowly in water forming a viscous solution; insoluble in ethanol, and ether |
| Precipitate formation with calcium chloride | To a 0.5% solution of the sample in sodium hydroxide TS add one-fifth of its volume of a 2.5% solution of calcium chloride. A voluminous, gelatinous precipitate is formed. This test distinguishes ammonium alginate from gum arabic, sodium carboxymethyl cellulose, carrageenan, gelatin, gum ghatti, karaya gum, carob bean gum, methyl cellulose and tragacanth gum. |
| Precipitate formation with ammonium sulfate | To a 0.5% solution of the sample in sodium hydroxide TS add one-half of its volume of a saturated solution of ammonium sulfate. No precipitate is formed. This test distinguishes ammonium alginate from agar, sodium carboxymethyl cellulose, carrageenan, de-esterified pectin, gelatin, carob bean gum, methyl cellulose and starch. |
| Test for alginate | Passes test |
| Ammonium | Passes test |
| PURITY | |
| Loss on drying | Not more than 15% (105o, 4 h) |
| Water-insoluble matter | Not more than 2% on the dried basisDisperse 2 g of the sample, weighed to the nearest 0.1 mg, in 800 ml of water in a 2,000-ml flask. Neutralize to pH 7 with sodium hydroxide TS and then add 3 ml in excess. Add 40 ml of hydrogen peroxide solution containing 30% by weight H2O2, cover the flask and boil for 1 h with frequent stirring. Filter while hot through a tared Gooch crucible provided with a glass fibre filter (2.4 cm, No. 934 AH, Reeve Angel & Co., Clifton, N.Y., or equivalent filter). If slow filtration is caused by high viscosity of the sample solution, boil until the viscosity is reduced enough to permit filtration. Wash the crucible thoroughly with hot water, dry the crucible and its contents at 105o for 1 h, cool and weigh. Calculate as percentage of the dry weight. |
| Sulfated ash | Not more than 7% on the dried basis |
| Lead | Not more than 2 mg/kgDetermine using an atomic absorption technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on the principles of the method described in Volume 4, “Instrumental Methods.” |
| Microbiological criteria | Total plate count: Not more than 5,000 colonies per gram.Initially prepare a 10-1 dilution by adding a 50 g sample to 450 ml of Butterfield’s phosphate buffered dilution water and homogenizing in a high speed blender.Yeasts and moulds: Not more than 500 colonies per gramColiforms: Negative by testSalmonella: Negative by test |
CyberColloids
