| Prepared at the 49th JECFA (1997) ,published in FNP 52 Add 5 (1997) superseding specifications prepared at the 44th JECFA (1995), published in FNP 52 Add 3 (1995). Metals and arsenic specifications revised at the 49th JECFA (1997). An ADI ‘not specified’ was established at the 39th JECFA (1992) | |
| SYNONYMS | INS No. 400 |
| DEFINITION | Alginic acid is a naturally occurring hydrophilic colloidal polysaccharide obtained from the various species of brown seaweed (Phaeophyceae). It is a linear copolymer consisting mainly of residues of ß-1,4-linked D-mannuronic acid and a-1,4-linked L-glucuronic acid. These monomers are often arranged in homopolymeric blocks separated by regions approximating an alternating sequence of the two acid monomers. |
| C.A.S. number | 9005-32-7 |
| Chemical formula | (C6H8O6)n |
| Structural formula | 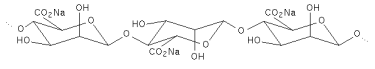 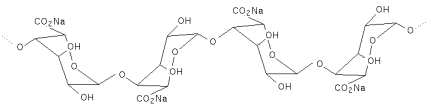 The number and sequence of the Mannuronate and Glucuronate residues shown above vary in the naturally occurring alginate. The associated water molecules are not shown. The number and sequence of the Mannuronate and Glucuronate residues shown above vary in the naturally occurring alginate. The associated water molecules are not shown. |
| Formula weight | Structural unit: 176.13 (theoretical), 200 (actual average)Macromolecule : 10,000 – 600,000 (typical average) |
| Assay | Yields, on the dried basis not less than 20.0% and not more than 23.0% of carbon dioxide (CO2), equivalent to not less than 91.0% and not more than 104.5% of alginic acid (C6H8O6)n . |
| DESCRIPTION | White to yellowish brown filamentous, grainy, granular or powdered forms |
| FUNCTIONAL USES | Stabilizer, thickener, gelling agent, emulsifier |
| CHARACTERISTICS | |
| IDENTIFICATION | |
| Solubility | Insoluble in water and organic solvents; dissolves slowly in solutions of sodium carbonate, sodium hydroxide and trisodium phosphate |
| pH | 2.0-3.5 (0.3 in 10 suspension) |
| Precipitate formation with with ammonium sulfate | To a 0.5% solution of the sample in sodium hydroxide TS add one-half of its volume of a saturated solution of ammonium sulfate. No precipitate is formed. This test distinguishes alginic acid from agar, sodium carboxymethyl cellulose, carrageenan, de-esterified pectin, gelatin, carob bean gum, methyl cellulose and starch. |
| Test for alginate | Passes testDissolve as completely as possible 0.1 g of sample by shaking with 0.15 ml of 0.1 N sodium hydroxide and add 1 ml of acid ferric sulfate TS. Within 5 min, a cherry-red colour develops that finally becomes deep purple. |
| PURITY | |
| Loss on drying | Not more than 15% (105o, 4 h) |
| Sulfated ash | Not more than 8% on the dried basis |
| Sodium hydroxide insoluble matter | Not more than 2% on the dried basisWeigh accurately about 1 g of the sample and dissolve in 100 ml of sodium hydroxide TS, centrifuge and decant. Wash the residue five times with water by mixing, centrifuging and decanting. Transfer the residue by means of water to a tared fine glass filter, dry for 1 h at 105o, cool and weigh. Calculate as percentage of the dry weight. |
| Arsenic | Not more than 3 mg/kg (Method II) |
| Lead | Not more than 5 mg/kgDetermine using an atomic absorption technique appropriate to the specified level. The selection of sample size and method of sample preparation may be based on the principles of the method described in Volume 4, “Instrumental Methods.” |
| Microbiological criteria | Total plate count: Not more than 5,000 colonies per gram.Initially prepare a 10-1 dilution by adding a 50 g sample to 450 ml of Butterfield’s phosphate buffered dilution water and homogenizing in a high speed blender.Yeasts and moulds: Not more than 500 colonies per gramColiforms: Negative by testSalmonella: Negative by test |
| METHOD OF ASSAY | Proceed as directed under Carbon Dioxide Determination by Decarboxylation in the General Methods. Each ml of 0.25 N sodium hydroxide consumed is equivalent to 5.5 mg of carbon dioxide (CO2) or 25 mg of alginic acid (equivalent weight 200). |
CyberColloids
